

The antimony sulfide ore was first ground in a pestle and mortar, after which it was mixed with oils or animal fats. Women of Egypt, Arabia, Ancient Greece, as well as the Romans found it fashionable to underline the eyes with this black cosmetic preparation made of antimony sulfide. They knew this substance under the Arabic name ‘ kohl ’, meaning ‘black powder’. People were powdering sulfide minerals containing antimony and have used it both as a medicine and in cosmetics ever since ancient times. When exposed to newly formed hydrogen, antimony may form stibine (SbH 3 ). The atomic structure of antimony is relatively stable in dry air, and this chalcophile is unreactive when exposed to dilute acids or alkalis.
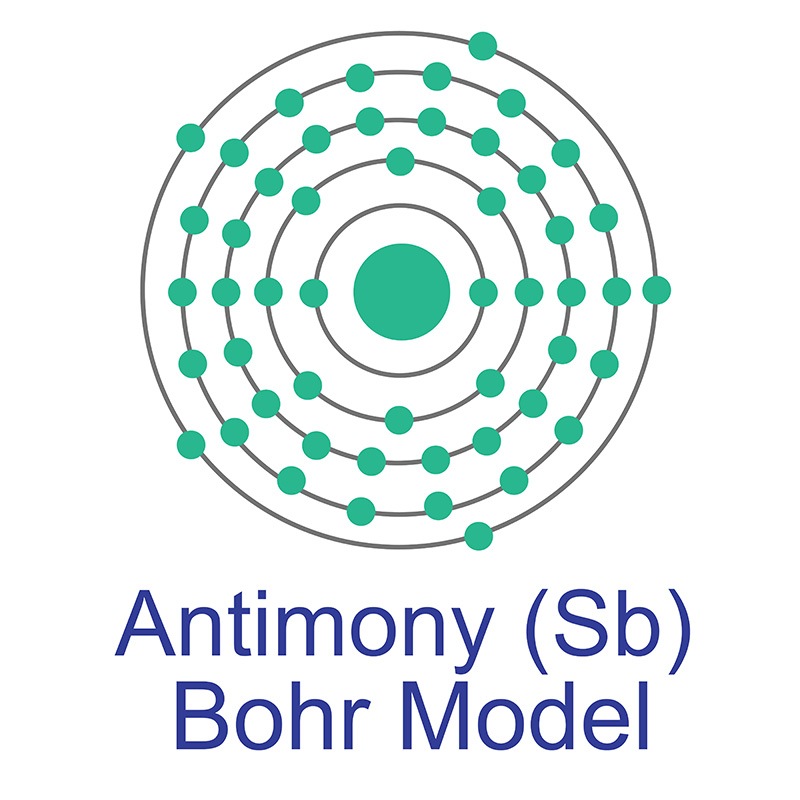
The shell structure of element 51 is 2.8.18.18. While the antimony metal is a very brittle, silvery, moderately hard metal, the non-metallic form is a grey powder. The chemical properties of this chemical are similar to the elements lead, arsenic, and bismuth.Īntimony can exist in two aggregate states.

This member of the nitrogen group of elements in the periodic table has an electronegativity of 1.9 according to Pauling, whereas the atomic radius according to van der Waals is 0.159 nm. With the chemical symbol Sb, atomic number 51, atomic mass of 121.75 g.mol-1, and electron configuration 4d105s25p3, аntimony is a semi-metallic chemical element. The energy of the third ionization: 2443 kJ.mol -1ĭiscovery date: Around 1600 BC by the ancient civilizations / Nicolas Lémery The energy of the second ionization: 1595 kJ.mol -1 The energy of the first ionization: 834 kJ.mol -1 Half-life: From less than 1.5 milliseconds to 2.75856 yearsĮlectronegativity according to Pauling: 1.9 Physical state: Solid at room temperature The symbol in the periodic table of elements: SbĬolor: A lustrous, silvery, bluish-white metalloid Fact Box Chemical and Physical Properties of Antimony


 0 kommentar(er)
0 kommentar(er)
